canion effect:
Li, Na, K, Cs
C---------O
smaller canion like Li can chelate with Oxygen, so carbon is more active compared to the oxygen atom. But at the same time, both C and O are less reactive compared to bigger canion!
solvent effect:
hexane, toluene, THF, acetone, DMF, DMSO
C----------------------O
substrate is less solvated in less polar solvent.
temperature
high temp seems favor O alkylation.
leaving group
OTs, Br, I
soft leaving group favors C-alkylation.
Friday, August 31, 2007
Saturday, August 25, 2007
a good article about research
1.一半时间做实验,一半时间看文献。half time doing experiments, half time reading!
千万不能把时间全部消耗在实验台上。看文献、看书、看别人的操作、听别人的经验、
研究别人的思路,边做边思考。要学会比较,不要盲从。否则,会被一些小小的问题困
扰许久。
2.准备越充分,实验越顺利。better preparation makes your experiment better.
古人云,磨刀不误砍柴工。前期的知识储备、文献储备、材料准备、方法准备可以避免
手忙脚乱,充分的预实验使你充满信心。一步一个脚印,就不必“从头再来”。最不能
容忍的是在开始的几步偷懒,造成后面总有一些无法排除的障碍。
3.记录真实详尽。record everything.
人总是有一点虚荣心的。只把成功的步骤或漂亮的结果记到实验记录里,是很多人的做
法。殊不知,许多宝贵经验和意外发现就这样与你擦肩而过。客观、真实、详尽的记录
是一笔宝贵的财富。
4.不要为老板省钱。Don't try to save money for your boss!
效率为先。整天算计着省钱,一旦用了不可靠的东西,只会浪费时间,遭受打击,到头
来一分钱也省不了。
5.把握心理优势。Keep your mind optimistic.
做过实验的人都经历过失败和挫折。有些失败应当在预实验阶段发生,你这时能坦然接
受。假如不做预实验,在正式的实验中遇到,你的挫折感就很明显。假如你因为赶时间
而误操作,你会沮丧。假如你能因为目前心浮气燥而果断地放一放,就可以避免悲剧的
发生。假如你早上进入实验室之前还不知道今天要干什么,你最好想好了再去。最大的
错误是重复犯同样的错误。记住,屡教不改者不适合做实验。
6.先看综述,后看论著. Read reviews first, then articles.
看综述搞清概念,看论著掌握方法
10.两手准备. choose research area which can be published even you get negtive result.
设计课题要为了阐明问题,即不论结果为阳性或阴性,都能写文章。阳性结果说明什么
,阴性结果说明什么。假如课题要求得出阳性结果,你可能要事先设计几部分,万一第
一部分得不出预期结果,可以用其它部分弥补损失。
14.准备引用的文章要亲自看过。Read every paper cited by you.
转引造成的以讹传讹不胜枚举。
17.交流是最好的老师. communication is the best teacher.
做实验遇到困难是家常便饭。你的第一反应是什么?反复尝试?放弃?看书?这些做法
都有道理,但首先应该想到的是交流。对有身份的人,私下的请教体现你对他的尊重;
对同年资的人,公开的讨论可以使大家畅所欲言,而且出言谨慎。千万不能闭门造车。
一个实验折腾半年,后来别人告诉你那是死路,岂不冤大头?
18.最高层次的能力是表达能力. expression of your self is a top ability.
再好的工作最终都要靠别人认可。表达能力,体现为写和说的能力,是需要长期培养的
素质。比如发现一个罕见病例,写好了发一篇论著;写不好只能发一个病例报道。比如
做一个课题,写好了发一篇或数篇论著;写不好只能发一个论著摘要或被枪毙。一张图
,一张表,无不是表达能力的体现。寥寥几百上千字的标书,可以赢得大笔基金;虽然关
系很重要,但写得太差也不行。有人说,我不学PCR,不学spss,只要学会ppt(
powerpoint)就可以了。此话有一点道理,实验室的boss 们表面上就是靠一串串ppt
行走江湖的。经常有研究生因思维敏捷条例清楚而令人肃然起敬。也经常有研究生不理
解“为什么我做了大部分工作而老板却让另一个没怎么干活的人写了文章?让他去大会
发言?”你没有看到人家有张口就来的本事吗?
千万不能把时间全部消耗在实验台上。看文献、看书、看别人的操作、听别人的经验、
研究别人的思路,边做边思考。要学会比较,不要盲从。否则,会被一些小小的问题困
扰许久。
2.准备越充分,实验越顺利。better preparation makes your experiment better.
古人云,磨刀不误砍柴工。前期的知识储备、文献储备、材料准备、方法准备可以避免
手忙脚乱,充分的预实验使你充满信心。一步一个脚印,就不必“从头再来”。最不能
容忍的是在开始的几步偷懒,造成后面总有一些无法排除的障碍。
3.记录真实详尽。record everything.
人总是有一点虚荣心的。只把成功的步骤或漂亮的结果记到实验记录里,是很多人的做
法。殊不知,许多宝贵经验和意外发现就这样与你擦肩而过。客观、真实、详尽的记录
是一笔宝贵的财富。
4.不要为老板省钱。Don't try to save money for your boss!
效率为先。整天算计着省钱,一旦用了不可靠的东西,只会浪费时间,遭受打击,到头
来一分钱也省不了。
5.把握心理优势。Keep your mind optimistic.
做过实验的人都经历过失败和挫折。有些失败应当在预实验阶段发生,你这时能坦然接
受。假如不做预实验,在正式的实验中遇到,你的挫折感就很明显。假如你因为赶时间
而误操作,你会沮丧。假如你能因为目前心浮气燥而果断地放一放,就可以避免悲剧的
发生。假如你早上进入实验室之前还不知道今天要干什么,你最好想好了再去。最大的
错误是重复犯同样的错误。记住,屡教不改者不适合做实验。
6.先看综述,后看论著. Read reviews first, then articles.
看综述搞清概念,看论著掌握方法
10.两手准备. choose research area which can be published even you get negtive result.
设计课题要为了阐明问题,即不论结果为阳性或阴性,都能写文章。阳性结果说明什么
,阴性结果说明什么。假如课题要求得出阳性结果,你可能要事先设计几部分,万一第
一部分得不出预期结果,可以用其它部分弥补损失。
14.准备引用的文章要亲自看过。Read every paper cited by you.
转引造成的以讹传讹不胜枚举。
17.交流是最好的老师. communication is the best teacher.
做实验遇到困难是家常便饭。你的第一反应是什么?反复尝试?放弃?看书?这些做法
都有道理,但首先应该想到的是交流。对有身份的人,私下的请教体现你对他的尊重;
对同年资的人,公开的讨论可以使大家畅所欲言,而且出言谨慎。千万不能闭门造车。
一个实验折腾半年,后来别人告诉你那是死路,岂不冤大头?
18.最高层次的能力是表达能力. expression of your self is a top ability.
再好的工作最终都要靠别人认可。表达能力,体现为写和说的能力,是需要长期培养的
素质。比如发现一个罕见病例,写好了发一篇论著;写不好只能发一个病例报道。比如
做一个课题,写好了发一篇或数篇论著;写不好只能发一个论著摘要或被枪毙。一张图
,一张表,无不是表达能力的体现。寥寥几百上千字的标书,可以赢得大笔基金;虽然关
系很重要,但写得太差也不行。有人说,我不学PCR,不学spss,只要学会ppt(
powerpoint)就可以了。此话有一点道理,实验室的boss 们表面上就是靠一串串ppt
行走江湖的。经常有研究生因思维敏捷条例清楚而令人肃然起敬。也经常有研究生不理
解“为什么我做了大部分工作而老板却让另一个没怎么干活的人写了文章?让他去大会
发言?”你没有看到人家有张口就来的本事吗?
Thursday, August 23, 2007
Computer aid Rh(II) catalyst design and synthesis
before I enter the project, the first generation cat. Rh2Ln2 was already made successfully, can catalyze C-H insertion, high ee but very low yield. We believe the very big and interconnected ligands blocked the active site of the Rh metal and were the reason for the low reactivity.
In the 2nd generation cat. Rh2T2, the ligand was smaller and interconnected by less number of carbons than the first generation. The only problem is the synthesis of the ligand was not successful.
So we did a little change to the ligand, which made the synthesis easier. The ligand was synthesized by diels-alder reaction of Evan's chiral auxiliary. But the reaction between Rh2(TFA)4 and the ligand is not successful. polymer like material was resulted, poor solubility in almost all organic solvents. Poor reactivity toward C-H insertion.
The reason I think is that the two carboxyl group of the ligand connected with two molecular of Rh2 instead of one Rh2. So you get a big molecular, a polymer.
In the 2nd generation cat. Rh2T2, the ligand was smaller and interconnected by less number of carbons than the first generation. The only problem is the synthesis of the ligand was not successful.
So we did a little change to the ligand, which made the synthesis easier. The ligand was synthesized by diels-alder reaction of Evan's chiral auxiliary. But the reaction between Rh2(TFA)4 and the ligand is not successful. polymer like material was resulted, poor solubility in almost all organic solvents. Poor reactivity toward C-H insertion.
The reason I think is that the two carboxyl group of the ligand connected with two molecular of Rh2 instead of one Rh2. So you get a big molecular, a polymer.
Pd catalyzed rxn
Heck reaction
Pd/phosphine ligand. Although Pd(OAc)2 was used, Pd (0) is the real catalyst. pd mirror was formed on the wall of the reaction vessel when I did the reaction.
Pd(MeCN)Cl2 mediated cyclization of beta-ketoester was not successful in my hand. probably the reason is the unpure homemade cat.
Pd(OAc)2 catalyzed cyclization of TES enolate to alkenes.
Pd/phosphine ligand. Although Pd(OAc)2 was used, Pd (0) is the real catalyst. pd mirror was formed on the wall of the reaction vessel when I did the reaction.
Pd(MeCN)Cl2 mediated cyclization of beta-ketoester was not successful in my hand. probably the reason is the unpure homemade cat.
Pd(OAc)2 catalyzed cyclization of TES enolate to alkenes.
Wednesday, August 22, 2007
Indole synthesis
Fisher indole synthesis
started with N-substituted benzene, C-C bond is formed by an intramolecular rearrangement.
finished with elimination of NH3.
Neber-Taber indole synthesis
started with C-substituted benzene, C-N bond is formed by N* attack the benzene. finished with proton transfer from benzene to N.
The intermediate azirine is very stable and under elevated temp. or rt with some metal catalyst (rh dimer for example), the azirine smoothly rearranged to indole.
started with N-substituted benzene, C-C bond is formed by an intramolecular rearrangement.
finished with elimination of NH3.
Neber-Taber indole synthesis
started with C-substituted benzene, C-N bond is formed by N* attack the benzene. finished with proton transfer from benzene to N.
The intermediate azirine is very stable and under elevated temp. or rt with some metal catalyst (rh dimer for example), the azirine smoothly rearranged to indole.
Friday, August 17, 2007
Radical cyclization
In general, halides can be reduced to radical by aibn. Bu3SnH is used to reduce the radical to R-H.
1. Mn(OAc)3
Mn3+ oxidizes the enone form of a beta-ketoester. A stablized carbon/oxygen radical is formed,
then it cyclizes to a double bond, the secondary carbon radical then can be oxidized by Cu2+, gives a cation, then finally, cation eliminates to give a double bond.
thermodynamically most stable isomer is the major product.
LiCl can be used instead of Cu(OAc)2 to give a chloride.
For some hindered double bond, Mn/Cu or Mn/LiCl do not work. O2 in the air reacted with the initial radical and R-OOH was the final product. if LiCl was used, R-Cl was formed.
In some case, push away O2 by Argon instead of N2 gives better yield.
Mn/EtOH gives reduced product instead of oxidized product. only works when you formed a methyl radical after cyclization.
AcOH is a good solvent, MeCN is also OK.
2. Iodine transfer reaction
treat a beta-ketoester with LDA/I2 gives the iodide. then aibn/heat/benzene gives cyclized product.
This method worked better for hindered double bond than Mn,
AIBN along with some heat is enough to initiate the rxn.
Lewis acid (for ex. Mg(ClO4)2 ) can be used to catalyze the reaction.
gives thermodynamic product.
3. selenide transfer rxn.
in my case, starting material - beta ketoester phenylselenide is not stable on silica gel.
usually, UV light, lewis acid can catalyze the reaction.
1. Mn(OAc)3
Mn3+ oxidizes the enone form of a beta-ketoester. A stablized carbon/oxygen radical is formed,
then it cyclizes to a double bond, the secondary carbon radical then can be oxidized by Cu2+, gives a cation, then finally, cation eliminates to give a double bond.
thermodynamically most stable isomer is the major product.
LiCl can be used instead of Cu(OAc)2 to give a chloride.
For some hindered double bond, Mn/Cu or Mn/LiCl do not work. O2 in the air reacted with the initial radical and R-OOH was the final product. if LiCl was used, R-Cl was formed.
In some case, push away O2 by Argon instead of N2 gives better yield.
Mn/EtOH gives reduced product instead of oxidized product. only works when you formed a methyl radical after cyclization.
AcOH is a good solvent, MeCN is also OK.
2. Iodine transfer reaction
treat a beta-ketoester with LDA/I2 gives the iodide. then aibn/heat/benzene gives cyclized product.
This method worked better for hindered double bond than Mn,
AIBN along with some heat is enough to initiate the rxn.
Lewis acid (for ex. Mg(ClO4)2 ) can be used to catalyze the reaction.
gives thermodynamic product.
3. selenide transfer rxn.
in my case, starting material - beta ketoester phenylselenide is not stable on silica gel.
usually, UV light, lewis acid can catalyze the reaction.
C-H Insertion/cyclopropanation (Rh, Cu...)
1. C-H insertion -> cyclopentane/cyclohexane
 the biggest side product is the dimer ( two diazo ketone lost one N2). usually unstable carbenoid gives less dimer, for example carbenoid with two electron withdrawing group or the rhodium dimer have electron-withdrawing ligand( for ex. Rh2(pttl)4).
the biggest side product is the dimer ( two diazo ketone lost one N2). usually unstable carbenoid gives less dimer, for example carbenoid with two electron withdrawing group or the rhodium dimer have electron-withdrawing ligand( for ex. Rh2(pttl)4).
stablized carbenoid reacts slowly so more dimmer, but better selectivity. for example,
In some situation, cyclohexane is formed instead of cyclopentane, rh2(piv)4 gives best 6/5 ratio.
2. double bond inerstion -> cyclopropane
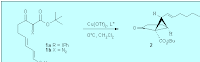
 The yield is usually higher than C-H inserstion. if the double bond is in a ring before the reaction, open the cyclopropane by hydrogenation will give CIS-ring fusion product.
The yield is usually higher than C-H inserstion. if the double bond is in a ring before the reaction, open the cyclopropane by hydrogenation will give CIS-ring fusion product.
3. O-H/N-H insertion.
Common catalysts:
hashimoto's cat.
rh2(pttl)4 , ptpa,....
very active toward intra. C-H insertion of ketones. higgest ee so far.
Davies' cat.
Rh2(DOSP)4, ...
intermolecular C-H or cyclopropanation.
intramolecular also ok.
Doyle's cat.
Rh2(MEOX)4 , mppim....
good for diazo esters. less reactive for diazoketones.
Taber's cat.
two ligands instead of four. ligand stability increased.
no good results so far.
DuBois's cat.
Rh2(ESP)2
very electron rich ligand. N-H insertion. derived from taber's cat.
diazo transfer reaction
ketone, beta-ketoester:
base: TEA, DBU
diazotransfer reagent: MsN3, AABSA, PNBSA, TIBSA
solvent: DCM, MeCN, Toluene
for a-aryl ketone, TIBSA/toluene/DBU works better.
for beta-ketoester, TEA/MeCN/MsN3 works OK. 1N NaOH can wash away excess MsN3.
ester:
Danheiser's method J. Org. Chem. 1990, 55, 1959.
introduced 2,2,2-trifluoroethyl trifluoroacetate and lithium bis(trimethylsilyl) amide as an efficient acylation method.
Taber's method
The TiCl4-mediated reaction of an ester with benzoyl chloride results in high yields of the R-benzoylated ester. Diazo transfer of the benzoylated ester utilizing p-acetoamidobenzenesulfonyl azide affords the R-diazo ester in good yield
acid->acid chloride then treated with diazomethane. TMSdiazomethane is a good substitution, but not in all cases. I did a reaction where TMSdiazomethane is not successful but diazomethane works.
ketone-> hydrazone->
 the biggest side product is the dimer ( two diazo ketone lost one N2). usually unstable carbenoid gives less dimer, for example carbenoid with two electron withdrawing group or the rhodium dimer have electron-withdrawing ligand( for ex. Rh2(pttl)4).
the biggest side product is the dimer ( two diazo ketone lost one N2). usually unstable carbenoid gives less dimer, for example carbenoid with two electron withdrawing group or the rhodium dimer have electron-withdrawing ligand( for ex. Rh2(pttl)4).stablized carbenoid reacts slowly so more dimmer, but better selectivity. for example,
In some situation, cyclohexane is formed instead of cyclopentane, rh2(piv)4 gives best 6/5 ratio.
2. double bond inerstion -> cyclopropane

 The yield is usually higher than C-H inserstion. if the double bond is in a ring before the reaction, open the cyclopropane by hydrogenation will give CIS-ring fusion product.
The yield is usually higher than C-H inserstion. if the double bond is in a ring before the reaction, open the cyclopropane by hydrogenation will give CIS-ring fusion product.3. O-H/N-H insertion.
Common catalysts:
hashimoto's cat.
rh2(pttl)4 , ptpa,....
very active toward intra. C-H insertion of ketones. higgest ee so far.
Davies' cat.
Rh2(DOSP)4, ...
intermolecular C-H or cyclopropanation.
intramolecular also ok.
Doyle's cat.
Rh2(MEOX)4 , mppim....
good for diazo esters. less reactive for diazoketones.
Taber's cat.
two ligands instead of four. ligand stability increased.
no good results so far.
DuBois's cat.
Rh2(ESP)2
very electron rich ligand. N-H insertion. derived from taber's cat.
diazo transfer reaction
ketone, beta-ketoester:
base: TEA, DBU
diazotransfer reagent: MsN3, AABSA, PNBSA, TIBSA
solvent: DCM, MeCN, Toluene
for a-aryl ketone, TIBSA/toluene/DBU works better.
for beta-ketoester, TEA/MeCN/MsN3 works OK. 1N NaOH can wash away excess MsN3.
ester:
Danheiser's method J. Org. Chem. 1990, 55, 1959.
introduced 2,2,2-trifluoroethyl trifluoroacetate and lithium bis(trimethylsilyl) amide as an efficient acylation method.
Taber's method
The TiCl4-mediated reaction of an ester with benzoyl chloride results in high yields of the R-benzoylated ester. Diazo transfer of the benzoylated ester utilizing p-acetoamidobenzenesulfonyl azide affords the R-diazo ester in good yield
acid->acid chloride then treated with diazomethane. TMSdiazomethane is a good substitution, but not in all cases. I did a reaction where TMSdiazomethane is not successful but diazomethane works.
ketone-> hydrazone->
Thursday, August 16, 2007
benzylic functionalization
1.super base (deprotonation directly)
t-BuOK/n-BuLi.
In hexanes, you get both deprotonation on benzylic and on the benzene ring, selectivity is bad.
Change the solvent to THF, only benzylic deprotonation resulted.
2. activated Zn + benzylic bromide
Zn can be activated by many methods, for example, TMSCl, dibromoethane, HCl or ultrasound, The activation reaction is hard to repeat.
other metal can be added to increase the reactivity of the benzylic anion.(Mn,Cu)
3. benzylic bromide + Mg (barbier type rxn)
works for some particular substrates only.
4. SmI2
ex. benzylic bromide + aldehyde.
barbier type, yield is only 50%, pivaldehyde can be added into the reaction mix to convert the alcohol to ketone in one pot.
using of Sm metal is also described in the literature.
5. Mn
t-BuOK/n-BuLi.
In hexanes, you get both deprotonation on benzylic and on the benzene ring, selectivity is bad.
Change the solvent to THF, only benzylic deprotonation resulted.
2. activated Zn + benzylic bromide
Zn can be activated by many methods, for example, TMSCl, dibromoethane, HCl or ultrasound, The activation reaction is hard to repeat.
other metal can be added to increase the reactivity of the benzylic anion.(Mn,Cu)
3. benzylic bromide + Mg (barbier type rxn)
works for some particular substrates only.
4. SmI2
ex. benzylic bromide + aldehyde.
barbier type, yield is only 50%, pivaldehyde can be added into the reaction mix to convert the alcohol to ketone in one pot.
using of Sm metal is also described in the literature.
5. Mn
Carbonyl umpolung
Wednesday, August 15, 2007
asymmetric epoxidation
Sharpless epoxidation
 allylic alcohol only.
allylic alcohol only.
homoallylic alcohol can also be reactive using some more active metal.
Jacobsen epoxidation
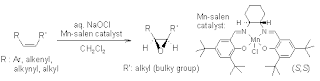
not limited to allylic alcohol, tetra-sub alkene gives low ee.
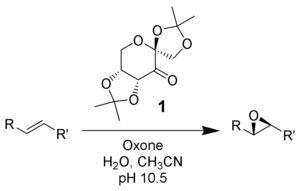
Shi epoxidation
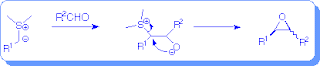
Sulfur Ylide Epoxidation
cordova's epoxidation
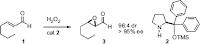 works for electron deficient olefins.
works for electron deficient olefins.
taber's mandelic acid approach based on column separation of two mandelic ester diasteromers.
based on column separation of two mandelic ester diasteromers.
Works on Terminal alkenes.
 allylic alcohol only.
allylic alcohol only.homoallylic alcohol can also be reactive using some more active metal.
Jacobsen epoxidation
not limited to allylic alcohol, tetra-sub alkene gives low ee.
Shi epoxidation

Sulfur Ylide Epoxidation

cordova's epoxidation
taber's mandelic acid approach
 based on column separation of two mandelic ester diasteromers.
based on column separation of two mandelic ester diasteromers.Works on Terminal alkenes.
Subscribe to:
Posts (Atom)

