The reduction by LAH on the following compound failed to give the desired product.
Instead, the allyl alcohol isolated.
So mechanismly, the hydride added to the double bond and the so-formed carbon anion caused the nitrile on the alpha position to eliminate.
But whether the ester is reduced before it or after is not clear now.
Thursday, July 14, 2011
Preparation of 1,2-diMagnesiummethylbenzene
The 1,2-dimagnesiummethylbenzene showing below is a useful reagent for metal ligand preparation.
This di-grignard reagent can be made in high yield from corresponding dichloride compound.
1,2-Dibromomethylbenzene can't be used because it can cause problem.
steps:
1. activate Mg using 1,2-dibromoethane.
2. in diluted solution, slowly add dichloride into Mg in THF. control temperature at rt.
3. allow 8 hr stirring at rt.
yield is over 95% in reports.
This di-grignard reagent can be made in high yield from corresponding dichloride compound.
1,2-Dibromomethylbenzene can't be used because it can cause problem.
steps:
1. activate Mg using 1,2-dibromoethane.
2. in diluted solution, slowly add dichloride into Mg in THF. control temperature at rt.
3. allow 8 hr stirring at rt.
yield is over 95% in reports.
Tuesday, April 5, 2011
Tuesday, March 1, 2011
an indole synthesis plan from nitrile
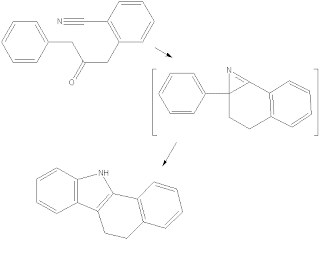
it is really plausible to me.
the additional six membered ring is required because without it, nitirle ylide will be formed instead of the azirine.
ps: sorry, the diazo compound is missing in the drawing.
Friday, January 28, 2011
preparation of thermodynamic silyl enol ethers
Tetrahedron Letters,Vol.24,No.l3,pp 1345-1348,1983
There now exist a variety of mild and very selective procedures for the kinetic
deprotonation of unsymmetrical ketones employing alkali metal dialkylamides. The "kinetic"
enolates produced in this way may be efficiently trapped by trimethylsilyl chloride to
regiospecifically provide the less substituted trimethylsilyl enol ethers.1 Despite the
recent introduction of several new methods, there are still no procedures available which
allow direct3 regiospecific preparation of the more substituted "thermodynamic" enolates or
trimethylsilyl enol ethers.
There now exist a variety of mild and very selective procedures for the kinetic
deprotonation of unsymmetrical ketones employing alkali metal dialkylamides. The "kinetic"
enolates produced in this way may be efficiently trapped by trimethylsilyl chloride to
regiospecifically provide the less substituted trimethylsilyl enol ethers.1 Despite the
recent introduction of several new methods, there are still no procedures available which
allow direct3 regiospecific preparation of the more substituted "thermodynamic" enolates or
trimethylsilyl enol ethers.
this paper described a way using MeMgBr/diisopropylamine to generate the thermodynamic enolate at rt, which worked out as described.(HMPA is necessary, without it, no rxn at all).
Friday, January 7, 2011
a mild alternative nitrene generation method for C-H amination
current method involve hypervalence iodine to oxidize the carbamate or sulfonamates to the imidoiodo species followed by metal incorperation to do following C-H aminations.
One problem limiting the rxn scope and catalyst preformance is the the high-oxidative potencial of the hypervalenc iodine compund such as pida, pifa.
here I propose a new oxidant which is a imidoiodo compound with a bulky blocking group attached to the imine. It is a milder oxidant than the pida/pifa and with a certain sized bulky group, the oxidant might be able to exchange its iodo with a carbamte but not able to react with the metal like rhodium.
Such a setup can increase the catalyst scope and performance and also milder the rxn condition.
One problem limiting the rxn scope and catalyst preformance is the the high-oxidative potencial of the hypervalenc iodine compund such as pida, pifa.
here I propose a new oxidant which is a imidoiodo compound with a bulky blocking group attached to the imine. It is a milder oxidant than the pida/pifa and with a certain sized bulky group, the oxidant might be able to exchange its iodo with a carbamte but not able to react with the metal like rhodium.
Such a setup can increase the catalyst scope and performance and also milder the rxn condition.
Subscribe to:
Posts (Atom)



