author:
Xinbo Wang, Bo Zhang and David Zhigang Wang
School of Chemical Biology and Biotechnology, Shenzhen Graduate School of Peking University, Shenzhen, China 518055
J. Am. Chem. Soc., Article ASAP
DOI: 10.1021/ja904224y
Publication Date (Web): July 21, 2009
In this recent paper, a novel oxidation of Secondary Alcohols by Sodium Hydride was described.
. they said "Uncovered here are some unprecedented reactivities of this classical reagent under very mild conditions, including alcohol oxidation, tandem allylic alcohol oxidation−hydride conjugate reduction, and aldehyde oxidative amidation. These readily implementable transition-metal-free processes feature exceptional material accessibility, operational simplicity, and environmental compatibility."
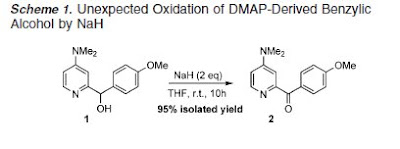
very nice work.
while reading this paper, some thoughts popped out of my little brain.
Could it be some other metal species instead of NaH really catalized the rxn?
it is known that nah is a base, sometimes can be a hydride donor, but Na+ never seen as a hydride acceptor.
I guess there is something else which catalized the rxn.
1. although nah is catalyst and all recoved after rxn, they still need a huge amount.
2. nah is made from na metal, presence of trans metal such as Pd is very possible.
3. there is an example by a japanese group which found a heck coupling rxn without pd, but finally they said the base they used (t-buOK, K2CO3?) contains trace of pd which did the trick.
(can't really remember all the detail, sorry).
anyway, this is still a very useful rxn and I hope to see more detailed research on this topic which may answer my question.
news:23,07,2009
http://totallysynthetic.com/blog/?p=1903
They already found O2 is essential for the rxn to happen.




